15 Days Free Returns*
15 Days Free Returns* 100% Authentic
100% Authentic Free Shipping
Free Shipping
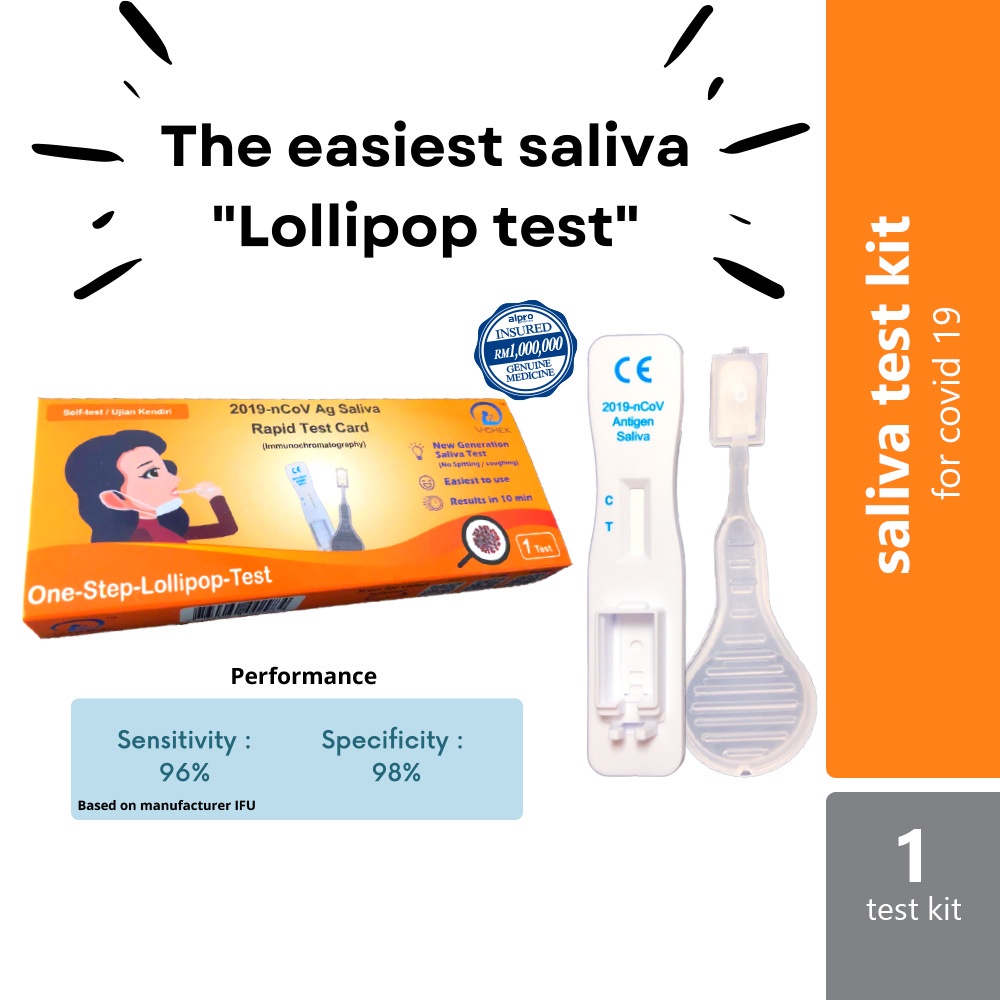
Saliva (lollipop) test
✅ No more painful sampling – easy and reliable antigentest from saliva (mouth fluids) swab.
✅ 95.65 % Sensitivity, 98.44 % Specificity
✅ Sample taken from mouth fluids (saliva)
✅ Fast result after 10 minutes
Brief Overview
The Test Card is a lateral flow immunoassay intended for the qualitative detection of
nucleocapsid protein antigen from 2019-nCoV in saliva specimens directly collected from
individuals who are suspected of COVID-19 by their healthcare provider within the first 7 days
of symptom onset.
Results are for the identification of 2019-nCoV nucleocapsid protein antigen. Positive results
indicate the presence of viral antigens, but clinical correlation with patient history and other
diagnostic information is necessary to determine infection status. Positive results do not rule
out bacterial infection or co-infection with other viruses. The agent detected may not be the
definite cause of disease.
Negative results should be treated as presumptive, and do not rule out 2019-nCoV infection
and should not be used as the sole basis for treatment or patient management decisions,
including infection control decisions. Negative results should be considered in the context of
a patient’s recent exposures, history, and the presence of clinical signs and symptoms
consistent with COVID-19, and confirmed with a molecular assay, if necessary, for patient
management.